AMORPHIZATION OF ZEOLITIC IMIDAZOLATE FRAMEWORKS
(ZIFs)

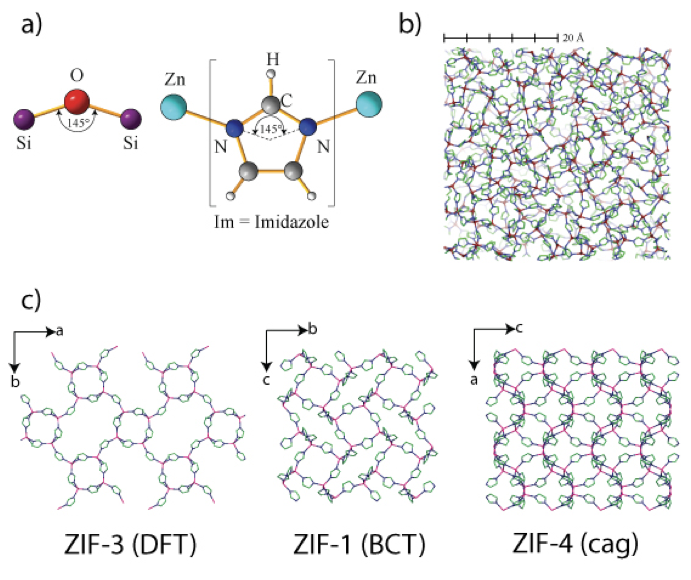
Figure showing a) the similarity in corner sharing inter-tetrahedral bond angle between ZIFs and silicate zeolites; b) the continuous random network based structure of amorphous ZIF, and c) the structures of three ZIFs, all of which undergo temperature and mechanical amorphization.
Zeolitic imidazolate frameworks (ZIFs) are strongly related to silica-based zeolites with their tetrahedral networks and similarity in their inter-tetrahedral bonding motif. They typically form larger structures with a metal-metal distance that is twice that of the Si-Si distance in silicates and there is scope for greater variety through variation of the imidazolate linker molecule. Working with colleagues from Tony Cheetham's group in Cambridge, we have observed that unsubstituted ZIFs such as ZIF-4 will amorphize on heating. Our neutron and x-ray scattering study shows that this amorphous phase is formed via a reconstructive transition and its structure is consistent with a silica glass continuous random network. The amorphous phase transforms to the more stable crystalline ZIF-zni phase on further heating [1,2].
Tom Bennett et
al then observed that seemingly the same amorphous phase could be
produced by mechanical means; ball milling crystalline ZIFs for relatively short
times leads to complete conversion to an amorphous product. X-ray total
scattering showed that these structures were identical to the structure of the
temperature-induced material [3]. Mechanosynthesis was found to be a more routine method for amorphous ZIF production and
it is also able to
produce amorphous ZIFs with substituted imidazolate linker ions.
More recently, glasses of ZIF-4 have been recovered from the melt by Tom
et al [4] and we have analysed the structure of the liquid phase using a
combination of RMC refinement of in-situ X-ray diffraction and first-principles
molecular dynamics simulation in collaboration with
F-X Coudert and
colleagues [5].
|
References
| 1. | Structure and properties of an amorphous metal-organic frameworkles | T D Bennett, A L Goodwin, M T Dove, D A Keen, M G Tucker, E R Barney, A K Soper, E G Bithell, J-C Tan and A K Cheetham | Phys. Rev. Lett. (2010) 104 115503 |
| 2. | Thermal amorphization of Zeolitic Imidazolate frameworks |
T D Bennett, D A Keen, J-C Tan, E R Barney, A L Goodwin, A K Cheetham |
Angew. Chem. Int. Ed. (2011) 50 3067 |
| 3. | Facile mechanosynthesis of amorphous zeolitic imidazolate frameworks | T D Bennett, S Cao, J C Tan, D A Keen, E G Bithell, P J Beldon, T Friscic and A K Cheetham | J. Am. Chem. Soc. (2011) 133 14546 |
| 4. | Melt-Quenched Glasses of Metal-Organic Frameworks | T D Bennett, Y Yue, P Li, A Qiao, H Tao, N G Greaves, T RIchards, G I Lampronti, S A T Redfern, F Blanc, O K Farha, J T Hupp, A K Cheetham and D A Keen | J. Am. Chem. Soc. (2016) 138 3484 |
| 5. | Liquid Metal-Organic Frameworks | R Gaillac, P Pullumbi, K A Bayer, K W Chapman, D A Keen, T D Bennett and F-X Coudert | Nature Mater. (2017) doi: 10.1038/NMAT4998 |
|
|